Samsung Biologics to Supply First Batch of Moderna Vaccines to Korea
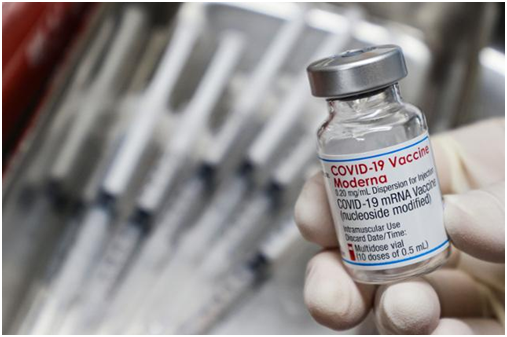
Moderna’s COVID-19 vaccine
A total of 2.43 million doses of Moderna’s COVID-19 vaccine produced by Samsung Biologics under an outsourcing agreement with Moderna will be released in Korea. A close collaboration between the government and the company moved up the vaccine shipment schedule by about four months.
The Ministry of Food and Drug Safety granted emergency use authorization (EUA) to the vaccine on Oct. 25, making the vaccines available at hospitals and clinics within this week.
Samsung Biologics signed a contract production deal with Moderna in May 2021 to provide fill-finish manufacturing for mRNA-1273, Moderna’s COVID-19 vaccine. Production began in August. It has recently completed production and secured enough volume for an initial shipment. Hundreds of millions of doses will be supplied to markets outside the United States by next year according to the contract.
A collaboration between the government and the private sector expedited production and supply of the vaccine. Samsung Electronics sent its smart factory team to Samsung Biologics to assist in facility automation and production process improvement. Samsung Biologics focused its company-wide capabilities on improving production yields. The government sped up the approval and shipment processes. As a result, the initial batch could be released in Korea four months earlier than originally expected.